Biofermin Pokkori Intestinal Chewable A 60 Tablets Taisho Pharmaceutical [Class 3 Drug]
¥2299
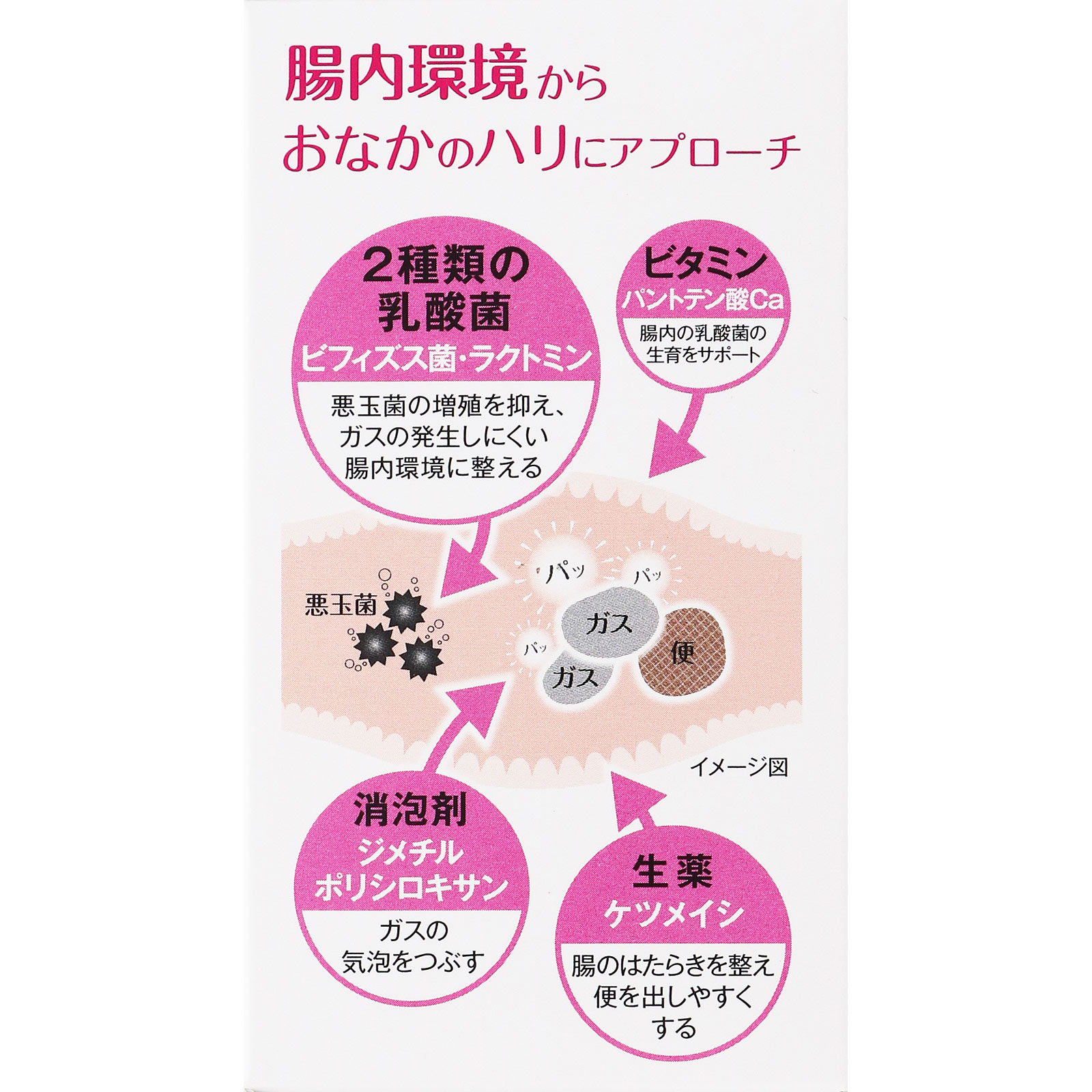
Improves stomach firmness caused by gas.
Yogurt flavor that can be chewed without water.
Efficacy/effect
-
Ingredients/Amount
Bifidobacterium 30mg, Lactomin 30mg, Ketumeishi extract 120mg (extracted from about 1,200mg Ketsumeishi), Dimethylpolysiloxane 180mg, Calcium pantothenate type S 34.6mg (22.5mg as calcium pantothenate)
Additives: Corn starch, erythritol, silicic anhydride, cellulose, reduced maltose starch syrup, candy flour, white sugar, hydroxypropyl cellulose, ascorbic acid, CMC-Ca, Mg stearate, Ca lactate, sucralose, aspartame (L-phenylalanine compound), fragrance
Usage and dosage
Please chew or dissolve in your mouth the following amount at least 4 hours apart.
(1) Please strictly follow the directions and dosage.
(2) Be sure to chew or dissolve this drug in your mouth before taking it.
(If you swallow it whole, it may get stuck in your throat.)
Dosage form/shape
Tablets
Usage precautions
Please chew or dissolve in your mouth the following amount at least 4 hours apart.
●1 tablet at a time for ages 15 and over, 3 times a day
●Do not use under the age of 15
Consultations regarding use
1. The following people should consult a doctor, pharmacist, or registered salesperson before taking the drug.
(1) Persons receiving treatment from a doctor.
(2) Persons who have received the following diagnosis.
Phenylketonuria
2. If your symptoms do not improve after taking this medicine for about 2 weeks, please stop taking it and consult your doctor, pharmacist, or registered salesperson.
Precautions for storage and handling
(1) Store tightly closed in a cool, dry place away from direct sunlight.
(2) Please keep out of reach of children.
(3) Do not transfer to another container.
(This may cause misuse or change in quality.)
(4) Please throw away the filling inside the container after opening the lid.
(If you put the filling back into the container, it will contain moisture and the quality will change.
The padding is to prevent the tablet from breaking during shipping. )
(5) Tightly close the lid of the container each time you take it.
(Other odors may be transferred or moisture may be absorbed, resulting in a change in quality.)
(6) Do not take products that have passed their expiration date.
(7) Please write the date on which the container was opened in the «Date of opening» field on the box and container.
(8) Once opened, please take the medicine as soon as possible, preferably within 6 months from the date of opening, in order to maintain quality.
Contact information
Taisho Pharmaceutical Co., Ltd.
3-24-1 Takada, Toshima-ku, Tokyo
Customer room 119: 03-3985-1800
Product size
Height 103mm x Width 56mm x Depth 56mm
| Габариты | 5.6 × 5.6 × 10.3 см |
|---|---|
| Medicine Category |
3 |
| Brand |
Biofermin |
| Manufacturer |
Taisho Pharmaceutical |
Before using this medicine, be sure to tell your doctor and pharmacist
・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
・If you are a patient with aspirin-induced asthma, or have a history of aspirin-induced asthma.
・If you have a history of hypersensitivity to tiaprofenic acid, suprofen, fenofibrate, or products containing oxybenzone or octocrylene (sunscreen, fragrance, etc.).
・If you have a history with photosensitivity reaction.
・If you are a patient with bronchial asthma.
・If you are under the age of 20 (If you have many opportunities to be exposed to sunlight such as outdoor activities, etc.).
・If you are pregnant or breastfeeding.
・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Precautions while taking this medicine
・Shield the application site from sunlight with colored clothes or supporters during application and for the time being after use, since this medicine may cause photosensitivity (a rash developed at the site exposed to sunlight).