New Mightia A 15Ml Daiichi Sankyo Healthcare [Category 3 Drug]
¥944
●A lacrimal type eye drop that is easy to apply and replenishes the important moisture of tears to the eyes and improves eye fatigue caused by dry eyes.
●It has a viscosity similar to tears, stabilizes the tear film that covers the surface of the cornea, and improves dryness of the eyes.
●Contains glucose, a nutritional ingredient found in tear fluid, which promotes eye metabolism and is effective for tired eyes.
●Eye drops that resemble tear fluid.
1. The ion balance of sodium, potassium, and calcium has been brought closer to normal tear fluid.
2. Contains calcium, which is important for maintaining corneal function and transparency.
3. The pH, osmotic pressure ratio, and viscosity have been brought closer to normal tear fluid.
●Colorless and clear eye drops.
Do not let the tip of the container touch your eyes, eyelids, or eyelashes.
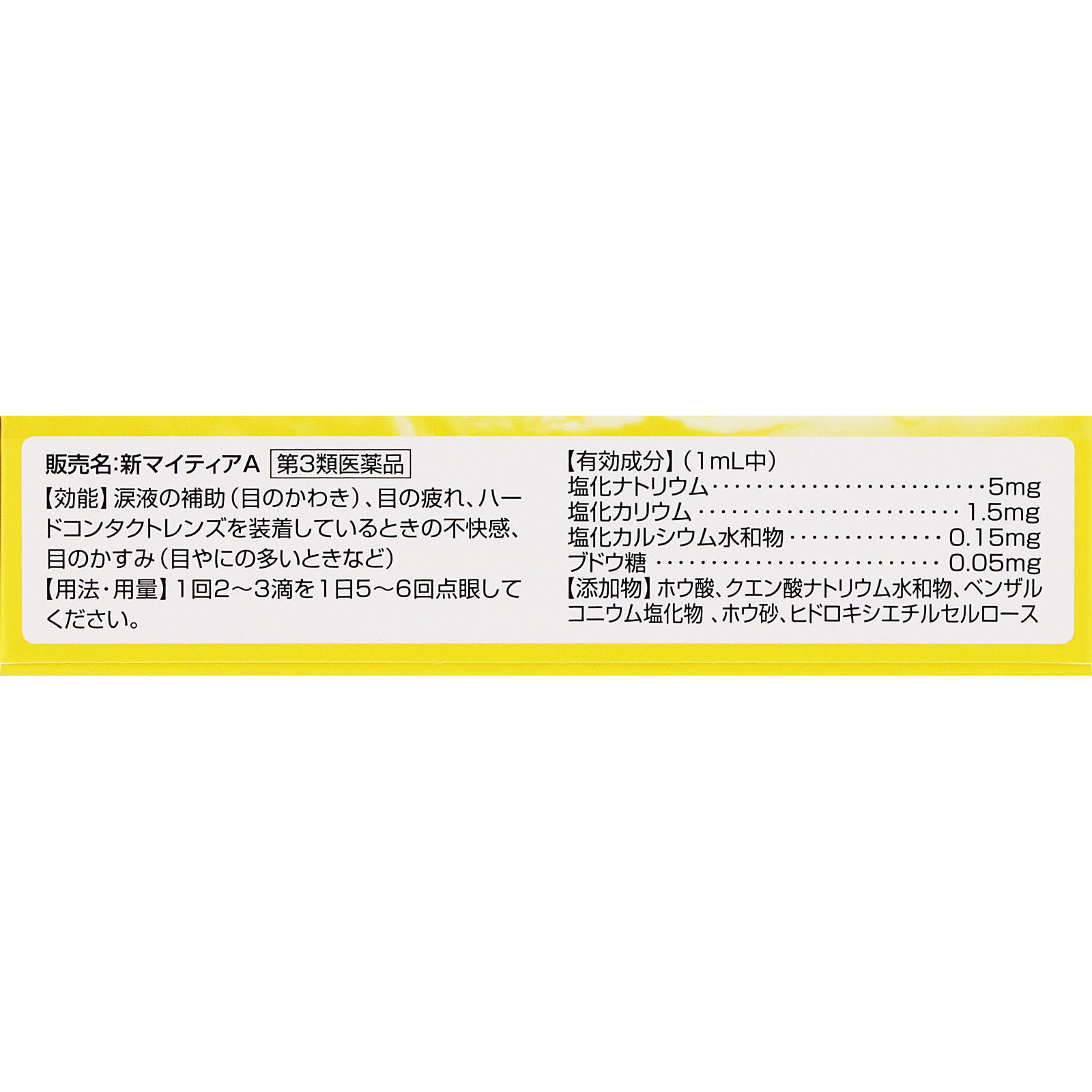
Efficacy/effect
Tear fluid support (dry eyes), eye fatigue, discomfort when wearing hard contact lenses, blurred vision (when there is a lot of eye mucus, etc.)
Ingredients/Amount
In 1mL
Sodium chloride…5mg
Potassium chloride…1.5mg
Calcium chloride hydrate…0.15mg
Glucose…0.05mg
Additives: boric acid, sodium citrate hydrate, benzalkonium chloride, borax, hydroxyethyl cellulose
Usage and dosage
Instill 2-3 drops at a time, 5-6 times a day.
(1) When allowing children to use this product, it must be used under the guidance and supervision of their guardians.
(2) Do not let the tip of the container touch your eyes, eyelids, or eyelashes (the drug solution may become contaminated or cloudy due to eye mucus or other foreign matter getting into it).
Also, do not use anything that is cloudy.
(3) Do not use soft contact lenses while wearing them.
(4) Use only for eye drops.
(5) Strictly follow the directions and dosage.
Dosage form/shape
Eye drops
Usage precautions
■Consultation
1. The following people should consult a doctor, pharmacist, or registered seller before use.
(1) Persons receiving treatment from a doctor.
(2) People who have had allergic symptoms due to drugs, etc.
(3) People with the following symptoms.
severe eye pain
(4) Persons who have received the following diagnosis.
glaucoma
2. If the following symptoms appear after use, it may be a side effect, so stop using it immediately and consult your doctor, pharmacist, or registered salesperson with this document.
[Related area: Symptoms]
Skin: Rash/redness, itching
Eyes: redness, itching, swelling, irritation
3. In the following cases, stop using the product and consult a doctor, pharmacist, or registered salesperson with this document.
(1) If blurred vision does not improve
(2) If the symptoms do not improve even after using it for about 2 weeks
Precautions for storage and handling
(1) Store tightly closed in a cool place away from direct sunlight. In particular, do not leave it in places where it may become hot, such as inside a car or near a heater.
(2) Store out of reach of children.
(3) Do not transfer to another container.
(It may cause misuse or change the quality.)
(4) Do not use the container with other items.
(5) Do not share with others.
(6) Do not use products that have passed their expiration date.
In addition, even if the expiration date has not passed, use the product immediately after opening the inner bag.
(7) Depending on storage conditions, crystals of ingredients in the drug solution may form around the tip of the container or inside the cap. In such cases, wipe it gently with clean gauze before use.
Contact information
Senju Pharmaceutical Co., Ltd.
541-0048 3-1-9 Kawaramachi, Chuo-ku, Osaka-shi, Osaka Prefecture
0120149931
Product size
Height 97mm x Width 58mm x Depth 23mm
| Габариты | 2.3 × 5.8 × 9.7 см |
|---|---|
| Medicine Category |
3 |
| Brand |
Mighty |
| Manufacturer |
Daiichi Sankyo Healthcare |
Before using this medicine, be sure to tell your doctor and pharmacist
・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
・If you are a patient with aspirin-induced asthma, or have a history of aspirin-induced asthma.
・If you have a history of hypersensitivity to tiaprofenic acid, suprofen, fenofibrate, or products containing oxybenzone or octocrylene (sunscreen, fragrance, etc.).
・If you have a history with photosensitivity reaction.
・If you are a patient with bronchial asthma.
・If you are under the age of 20 (If you have many opportunities to be exposed to sunlight such as outdoor activities, etc.).
・If you are pregnant or breastfeeding.
・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Precautions while taking this medicine
・Shield the application site from sunlight with colored clothes or supporters during application and for the time being after use, since this medicine may cause photosensitivity (a rash developed at the site exposed to sunlight).