Okuda Neurological Agent 50 Tablets Okuda Pharmaceutical [Designated Class 2 Drug]
¥2396
This is an easy-to-swallow sedative tablet containing Japanese, Chinese, and Western drugs that are effective for sedation and analgesia necessary to suppress neurological symptoms.
Efficacy/effect
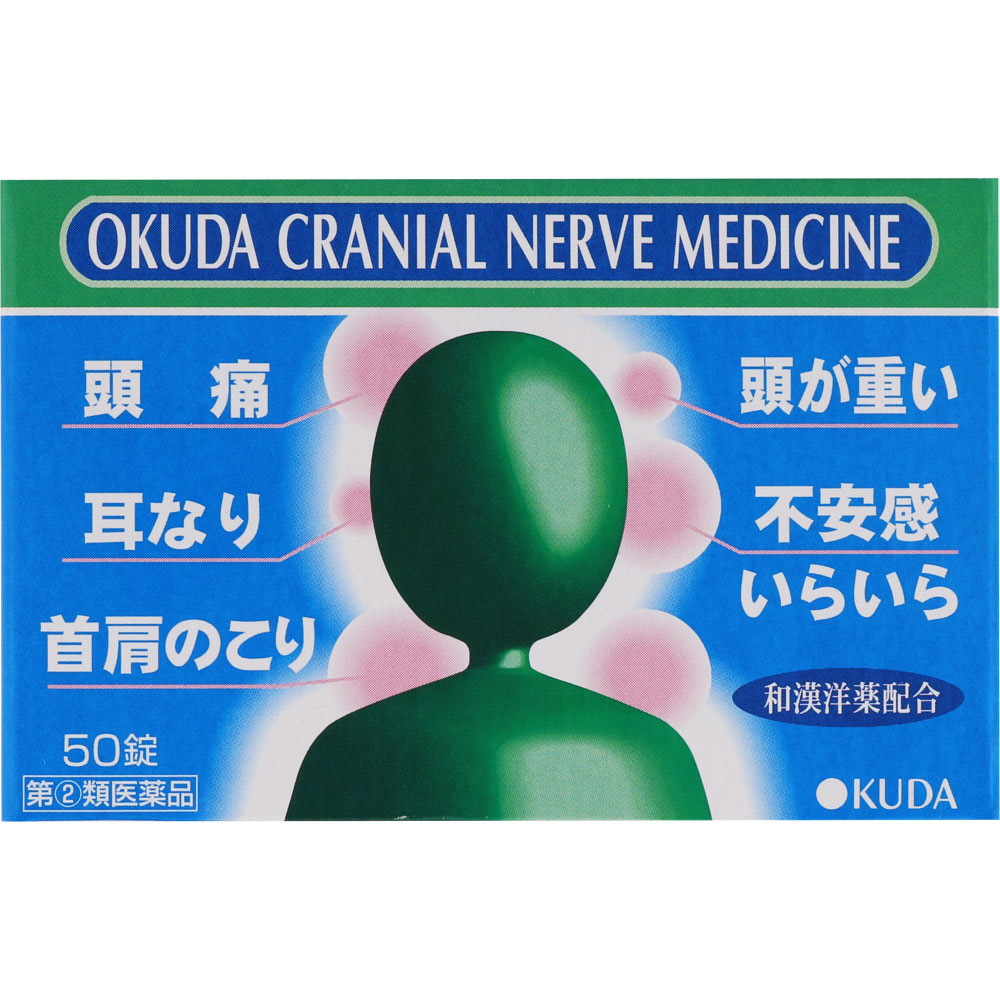
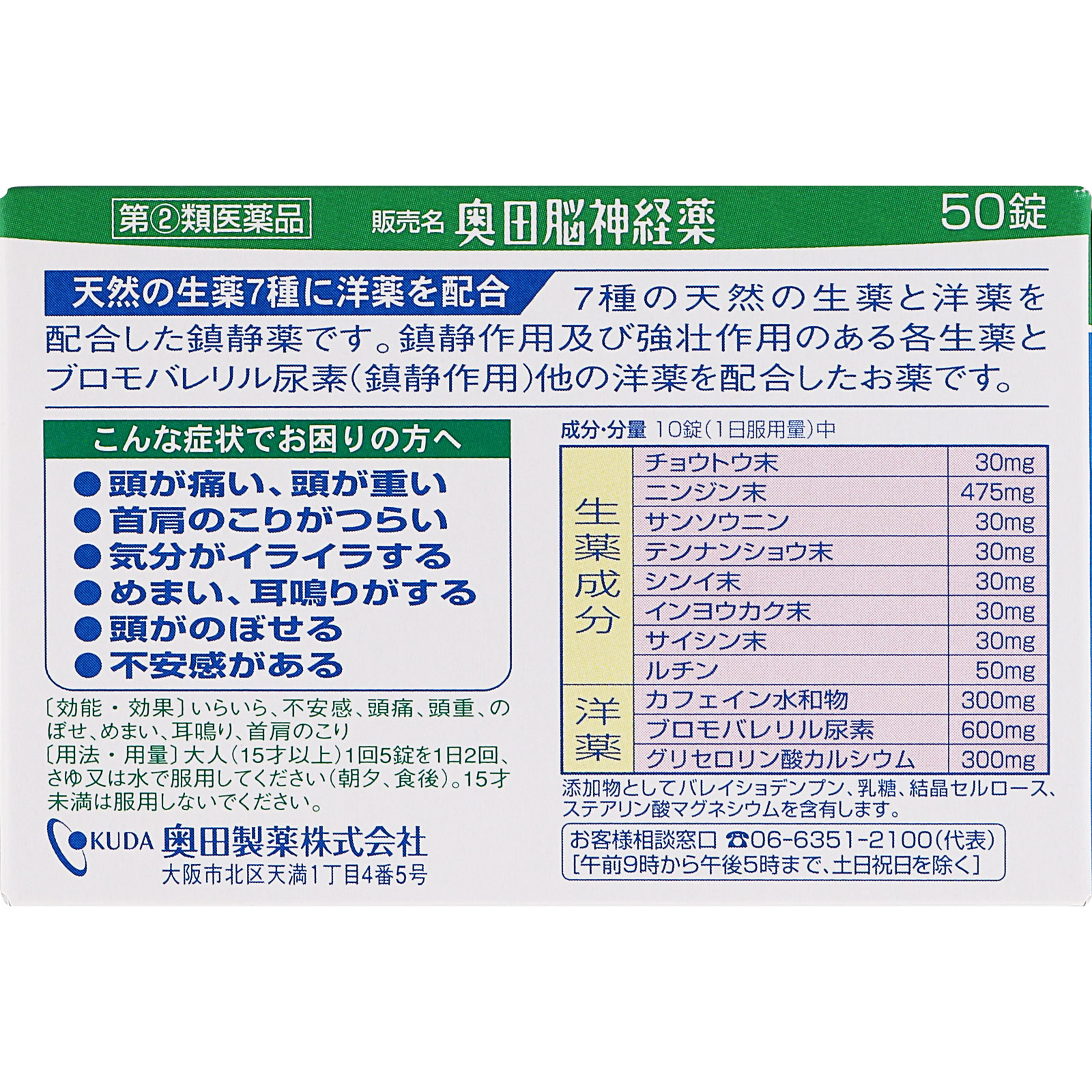
Irritability, anxiety, headache, headache, hot flashes, dizziness, ringing in the ears, stiff neck and shoulders
Ingredients/Amount
[Ingredients]
10 tablets (daily dose)
Chotou end (chotou end)…30mg
Carrot end (carrot end)…475mg
Sansounin (acid dates)…30mg
Tennansho end (Tennan star end)…30mg
Shinyi powder (Shinyi powder)…30mg
Inyoukaku powder (Inyoukaku powder)…30mg
Saishin powder (spicy powder)…30mg
Rutin…50mg
Caffeine hydrate…300mg
Bromovaleryl urea…600mg
Calcium glycerophosphate…300mg
[Additives]
Contains potato starch, lactose, crystalline cellulose, and magnesium stearate.
Usage and dosage
Please take the following amount with soy sauce or water.
○Adults (15 years and older): 5 tablets per dose, 2 doses per day
○Those under 15 years old: Do not take this product.
1. Take it in the morning and evening, preferably after meals.
2. For some people, taking this medicine before bedtime may make it difficult to fall asleep, so for these people, we recommend taking the medicine 4 to 5 hours before bedtime instead of just before going to bed.
3. Please strictly follow the prescribed usage and dosage.
Dosage form/shape
Uncoated tablet
Usage precautions
1. The following people should not take this drug:
People who have had allergic symptoms due to this drug.
2. Do not take any of the following medicines while taking this drug:
Oral medicines containing other hypnotic sedatives, sedatives, cold medicines, antipyretic analgesics, antitussive expectorants, and antihistamines (oral medicines for rhinitis, medicines for motion sickness, medicines for allergies)
3. Do not drive a vehicle or operate machinery after taking this medicine (drowsiness may occur).
4. Do not drink alcohol while taking
5. Do not use it for a long time
Consultations regarding use
1. The following people should consult a doctor, pharmacist, or registered seller before use.
(1) Persons receiving treatment from a doctor.
(2) People who have had allergic symptoms due to drugs, etc.
(3) People with the following symptoms.
severe eye pain
(4) Persons who have received the following diagnosis.
glaucoma
2. If the following symptoms appear after use, it may be a side effect, so please stop using it immediately and consult your doctor, pharmacist, or registered seller with this document.
Related parts: Symptoms
Skin: Rash/redness, itching
Eyes: bloodshot, itching, swelling
3. In the following cases, stop using the product and consult your doctor, pharmacist, or registered salesperson with this document.
(1) When blurred vision is not improved.
(2) If the symptoms do not improve even after using the product for about 2 weeks.
Precautions for storage and handling
(1) Avoid direct sunlight and store in a cool, dry place.
(2) Store out of reach of children.
(3) Do not transfer to another container.
(To prevent misuse or change in quality.)
(4) If the product is in a bottle, please tightly close the bottle lid each time you take it.
(5) The filling inside the bottle is placed to prevent the tablet from breaking, so please throw it away after opening.
(6) Do not take the product after the expiration date. Please take the medicine as soon as possible after opening it, even if it is within the expiration date.
Contact information
Okuda Pharmaceutical Co., Ltd. Customer Service Desk
1-4-5 Tenma, Kita-ku, Osaka-shi, Osaka Prefecture
(06)6351-2100
Product size
Height 66mm x Width 100mm x Depth 25mm
| Габариты | 2.5 × 10 × 6.6 см |
|---|---|
| Medicine Category |
2 |
| Manufacturer |
Okuda Pharmaceutical |
Before using this medicine, be sure to tell your doctor and pharmacist
・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
・If you are a patient with aspirin-induced asthma, or have a history of aspirin-induced asthma.
・If you have a history of hypersensitivity to tiaprofenic acid, suprofen, fenofibrate, or products containing oxybenzone or octocrylene (sunscreen, fragrance, etc.).
・If you have a history with photosensitivity reaction.
・If you are a patient with bronchial asthma.
・If you are under the age of 20 (If you have many opportunities to be exposed to sunlight such as outdoor activities, etc.).
・If you are pregnant or breastfeeding.
・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Precautions while taking this medicine
・Shield the application site from sunlight with colored clothes or supporters during application and for the time being after use, since this medicine may cause photosensitivity (a rash developed at the site exposed to sunlight).