Ozo Children’S Cough Stop Syrup 120Ml Meiji Pharmaceutical [Designated Class 2 Drug]
¥1186
The package and bottle have illustrations of Doraemon, which children love.
Adopts strawberry flavor that children like. Adopts a safety cap to prevent accidental ingestion. No caffeine, no codeine. Contains crude drug ingredients.
Can be taken up to age 15.
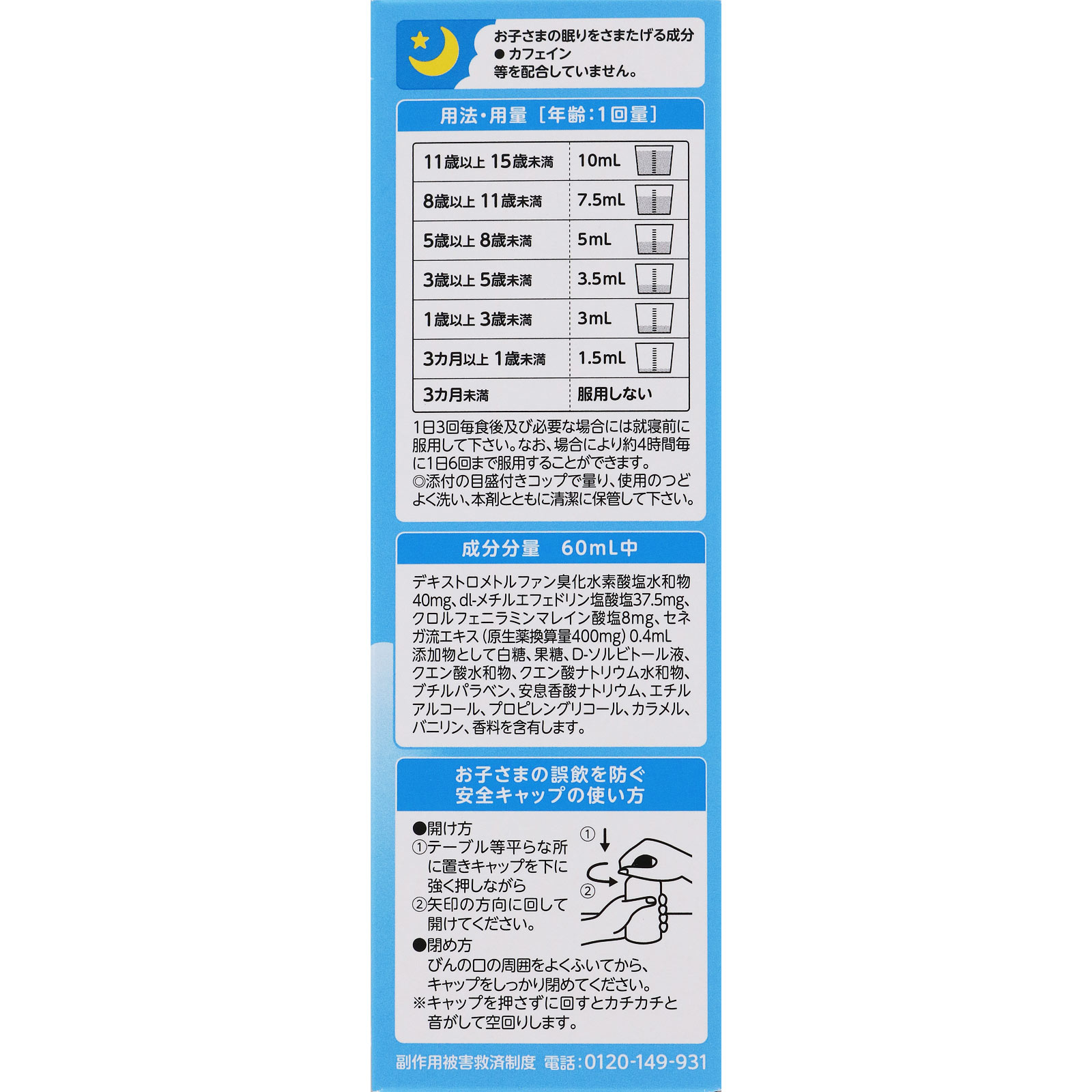
Contains 40mg of dextromethorphan, which has antitussive and expectorant effects.
Efficacy/effect
Cough and phlegm relief
Ingredients/Amount
Dextromethorphan hydrobromide hydrate 40mg/dl-methylephedrine hydrochloride 37.5mg/chlorpheniramine maleate 8mg/Senega flow extract (original drug equivalent amount 400mg) 0.4mL
Usage and dosage
Take 3 times a day after each meal and before bedtime if necessary. In some cases, the medicine may be taken up to 6 times a day, approximately every 4 hours.
11 years old or older and under 15 years old: 10mL per dose
8 years old or older and under 11 years old 7.5mL per dose
Ages 5 to 8: 5mL per dose
3 years old or older and under 5 years old: 3.5mL per dose
1 year old or older and under 3 years old: 3mL at a time
3 months or older and under 1 year old: 1.5mL once
Do not take it for less than 3 months
Dosage form/shape
Bottle
Usage precautions
1. The following people should not take this product:
People who have had allergic symptoms due to this drug or its ingredients.
2. Do not use any of the following medicines while taking this drug:
Other antitussive expectorants, cold medicines, sedatives, oral medicines containing antihistamines, etc. (oral medicines for rhinitis, motion sickness medicine, allergy medicines, etc.)
3. After taking this medicine, do not drive a vehicle or operate machinery (you may experience drowsiness, etc.)
Consultations regarding use
1. The following people should consult a doctor, pharmacist, or registered salesperson before taking the drug.
●Persons receiving treatment from a doctor.
●Pregnant women or people who appear to be pregnant.
●People who are breastfeeding.
●Elderly people.
●Persons who have had allergic symptoms due to medicines, etc.
●Persons with the following symptoms.
High fever, difficulty urinating
●Persons who have received the following diagnosis.
Heart disease, high blood pressure, diabetes, glaucoma, thyroid dysfunction
2. If the following symptoms appear after taking this medicine, it may be a side effect, so please stop taking it immediately and consult your doctor, pharmacist, or registered salesperson with this document.
Skin: Rash/redness, itching
Gastrointestinal: Nausea/vomiting, loss of appetite
Neuropsychiatric system: dizziness
Respiratory: shortness of breath, difficulty breathing
Urinary system: difficulty urinating
In rare cases, the following serious symptoms may occur. In that case, please see a doctor immediately.
shock (anaphylaxis)
Immediately after taking the drug, symptoms such as itchy skin, hives, hoarse voice, sneezing, itchy throat, difficulty breathing, palpitations, and clouded consciousness may occur.
aplastic anemia
Bruising, nosebleeds, bleeding gums, fever, pale skin and mucous membranes, fatigue, palpitations, shortness of breath, feeling unwell and dizzy, blood in the urine, etc. may appear.
agranulocytosis
Sudden onset of high fever, chills, sore throat, etc.
3. The following symptoms may appear after taking this medicine. If these symptoms persist or worsen, please stop taking this medicine and consult your doctor, pharmacist, or registered salesperson with this document.
Dry mouth, drowsiness
4. If your symptoms do not improve after taking 5 to 6 times, please stop taking the drug and consult your doctor, pharmacist, or registered salesperson with this document.
Precautions for storage and handling
1 Store tightly closed in a cool, dry place away from direct sunlight.
2. Keep out of reach of children.
3. Please do not transfer to another container.
(This may cause misuse or change in quality)
4. Wipe the area around the mouth of the bottle clean before each dose and close the cap.
5. Do not take the product after the expiration date.
6. Once you open the lid, please do not use it for a long period of time, even if it is within the expiration date.
Contact information
Meiji Pharmaceutical Co., Ltd.
6 Misato, Toyama City, Toyama Prefecture 939-3548
076-478-2990
Product size
Height 150mm x Width 48mm x Depth 48mm
Interior size
Height 150mm x Width 48mm x Depth 48mm
| Габариты | 4.8 × 4.8 × 15 см |
|---|---|
| Medicine Category |
2 |
| Brand |
Ozo |
| Manufacturer |
Meiji Pharmaceutical |
Before using this medicine, be sure to tell your doctor and pharmacist
・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
・If you are a patient with aspirin-induced asthma, or have a history of aspirin-induced asthma.
・If you have a history of hypersensitivity to tiaprofenic acid, suprofen, fenofibrate, or products containing oxybenzone or octocrylene (sunscreen, fragrance, etc.).
・If you have a history with photosensitivity reaction.
・If you are a patient with bronchial asthma.
・If you are under the age of 20 (If you have many opportunities to be exposed to sunlight such as outdoor activities, etc.).
・If you are pregnant or breastfeeding.
・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Precautions while taking this medicine
・Shield the application site from sunlight with colored clothes or supporters during application and for the time being after use, since this medicine may cause photosensitivity (a rash developed at the site exposed to sunlight).