Antidiarrheal Powder Powder 30 Tablets Kracie Pharmaceutical [Class 2 Drug]
¥845
●Berberine chloride hydrate and Gennoshoko extract help improve stomach health, and are effective against diarrhea, perishing food, perishing water, and loose stools.
●Film-coated tablets that are easy to take.
Efficacy/effect
Diarrhea, per food, per water, loose stools, diarrhea due to indigestion, drooling, drooping stomach
Ingredients/Amount
Adult daily dose of 6 tablets (1 tablet 155 mg)
Berberine chloride hydrate…225mg
Gennoshoko extract…600mg (equivalent to 4g of crude drug)
[Additives]
Contains light silicic anhydride, CMC-Ca, crystalline cellulose, synthetic Al silicate, Mg stearate, hypromellose, titanium oxide, yellow iron sesquioxide, and carnauba wax.
Usage and dosage
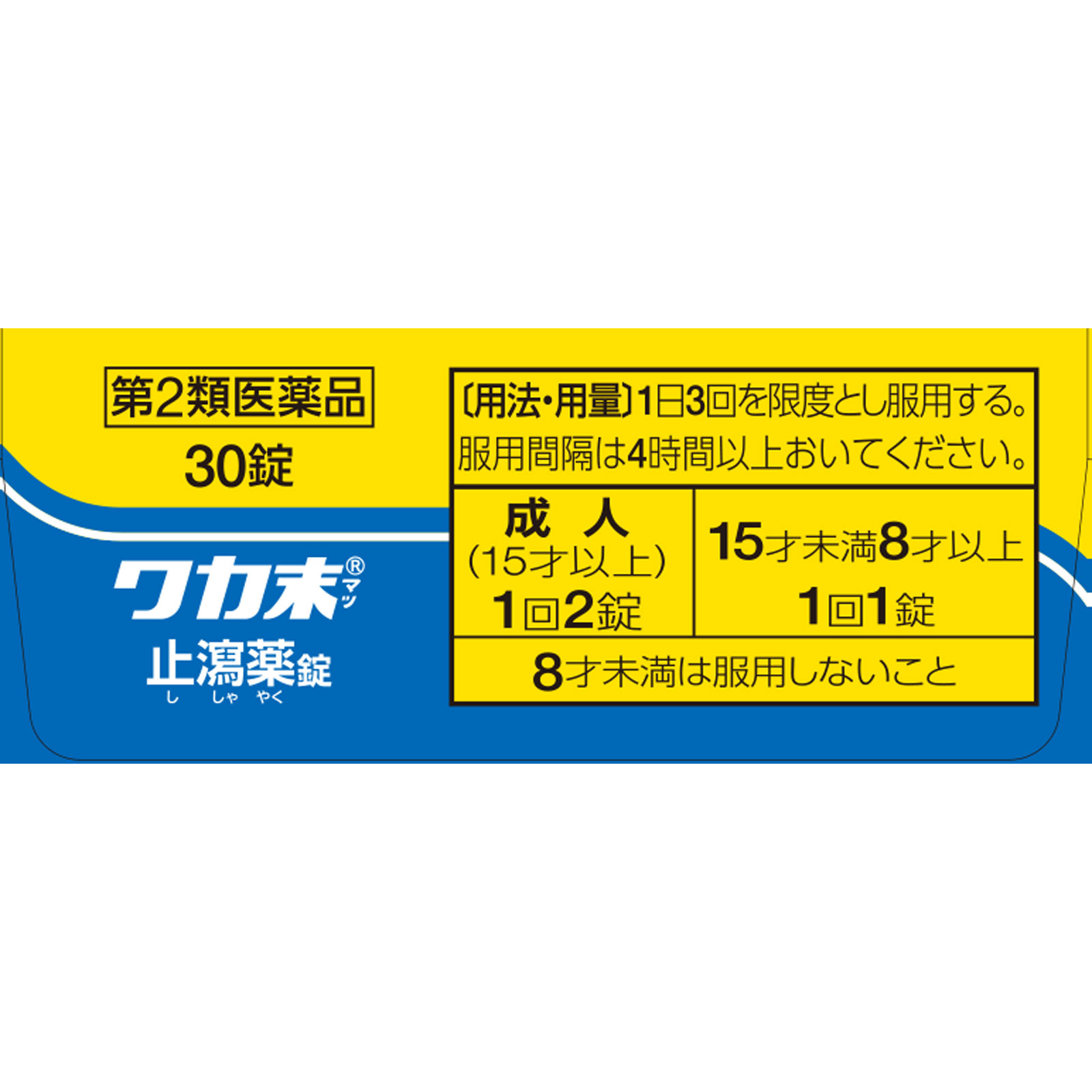
●Take the drug no more than three times a day.
●Please wait at least 4 hours between doses.
・Adults (15 years and older): 2 tablets at a time
・Under 15 years old and over 8 years old: 1 tablet at a time
・Do not use if under 8 years old
Dosage form/shape
Tablets
Usage precautions
(1) When giving this product to children, please use it under the guidance and supervision of their parents.
(2) How to take out the tablet
・Press firmly on the convex part of the PTP sheet containing the tablet to break the aluminum foil on the back, then take it out and take it.
(If you accidentally swallow it as is, it may pierce the esophageal mucosa or cause an unexpected accident.)
Consultations regarding use
● Consultation
1. The following people should consult a doctor, pharmacist, or registered salesperson before taking the drug.
(1) Persons receiving treatment from a doctor
(2) People with diarrhea accompanied by fever, bloody stools, or persistent mucus stools
(3) People who have acute severe diarrhea or diarrhea accompanied by symptoms such as abdominal pain, bloating, and nausea (forcibly stopping diarrhea with this drug may actually worsen the disease).
(4) Elderly people
2. If your symptoms do not improve after taking this medicine for 5 to 6 days, please stop taking it and consult your doctor, pharmacist, or registered salesperson with this document.
Precautions for storage and handling
(1) Store in a cool, dry place away from direct sunlight.
(2) Please keep out of reach of children.
(3) Do not transfer to another container.
(It may cause misuse or change in quality.)
(4) Do not take products whose expiration date has passed.
(5) If water gets on the tablet, it may cause discoloration or uneven coloring, so please do not accidentally drop water droplets on it or touch it with wet hands.
Contact information
Kuracie Pharmaceutical Co., Ltd.
3-20-20 Kaigan, Minato-ku, Tokyo 108-8080
(03)5446-3334
Product size
Height 65mm x Width 114mm x Depth 27mm
| Dimensions | 2.7 × 11.4 × 6.5 cm |
|---|---|
| Medicine Category |
2 |
| Manufacturer |
Kracie Pharmaceutical |
Before using this medicine, be sure to tell your doctor and pharmacist
・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
・If you are a patient with aspirin-induced asthma, or have a history of aspirin-induced asthma.
・If you have a history of hypersensitivity to tiaprofenic acid, suprofen, fenofibrate, or products containing oxybenzone or octocrylene (sunscreen, fragrance, etc.).
・If you have a history with photosensitivity reaction.
・If you are a patient with bronchial asthma.
・If you are under the age of 20 (If you have many opportunities to be exposed to sunlight such as outdoor activities, etc.).
・If you are pregnant or breastfeeding.
・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Precautions while taking this medicine
・Shield the application site from sunlight with colored clothes or supporters during application and for the time being after use, since this medicine may cause photosensitivity (a rash developed at the site exposed to sunlight).