Benzaace A Tablet 120T Alinamin Pharmaceutical [Designated Class 2 Drug]
¥2396
●A comprehensive cold medicine containing tranexamic acid, which suppresses sore throats and swelling, and hesperidin, a type of vitamin P found in citrus fruits.
●It is effective against sore throats and runny noses that often occur at the beginning of a cold.
●7 types of ingredients including acetaminophen, an antipyretic and analgesic ingredient, work in a well-balanced manner to alleviate various cold symptoms.
●It is a small, pale yellow tablet that is easy to take, and can be taken by the whole family (6 years old and older).
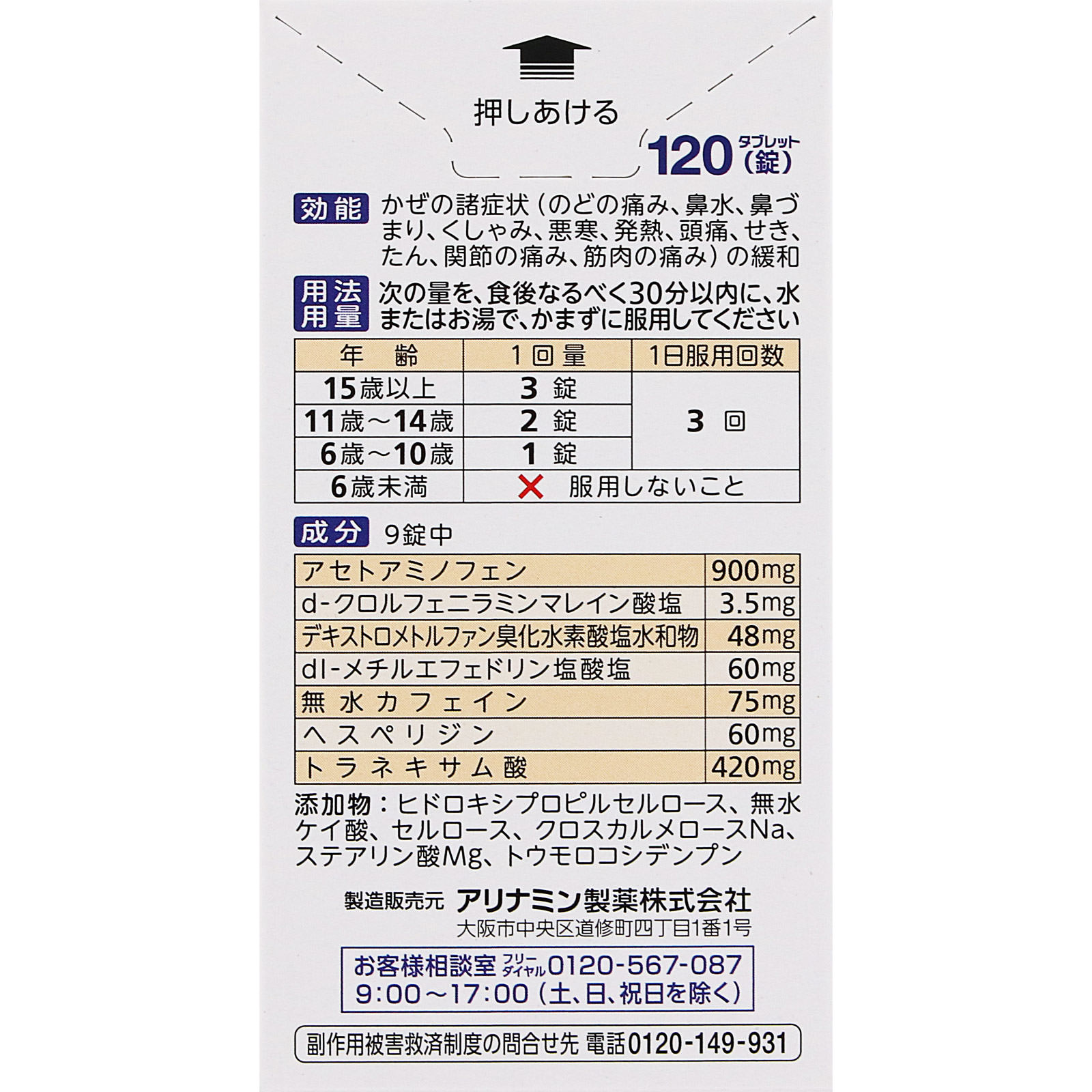
Efficacy/effect
Relief of cold symptoms (sore throat, runny nose, stuffy nose, sneezing, chills, fever, headache, cough, sputum, joint pain, muscle pain)
Ingredients/Amount
9 tablets (daily dose for people over 15 years old)
Acetaminophen…900mg
(Reduces fever and relieves pain)
d-Chlorpheniramine maleate…3.5mg
(Relieve runny nose and sneezing)
Dextromethorphan hydrobromide hydrate…48mg
(relieve cough)
dl-methylephedrine hydrochloride…60mg
(relieve cough)
Anhydrous caffeine…75mg
(relieve headache)
Hesperidin…60mg
(Vitamins (a type of vitamin P))
Tranexamic acid…420mg
(Relieve sore throat)
Additives: Hydroxypropyl cellulose, silicic anhydride, cellulose, croscarmellose Na, Mg stearate, corn starch
Usage and dosage
Take the following amount with water or hot water, without chewing, preferably within 30 minutes after meals.
Over 15 years old: 3 tablets per dose, 3 times per day
11 to 14 years old: 2 tablets per dose, 3 times per day
6 to 10 years old: 1 tablet at a time, 3 times a day
Children under 6 years of age: Do not take
(1) When administering this product to a child, it must be administered under the guidance and supervision of a guardian.
(2) Strictly follow the directions and dosage.
Dosage form/shape
Uncoated tablet
Usage precautions
1. The following people should not take this drug:
(1) Persons who have had allergic symptoms due to this drug or its ingredients.
(2) People who have had asthma while taking this drug or other cold medicines or antipyretic analgesics.
2. Do not use any of the following medicines while taking this drug:
Other cold medicines, antipyretic analgesics, sedatives, antitussive expectorants, oral medicines containing antihistamines (oral medicines for rhinitis, motion sickness medicines, allergy medicines, hypnotic sedatives, etc.), oral medicines containing tranexamic acid
3. Do not drive or operate machinery after taking this medicine.
(Drowsiness, etc. may occur.)
4. Do not drink alcohol before or after taking the drug
5. Do not use it for a long time
Consultations regarding use
1. The following people should consult a doctor, pharmacist, or registered salesperson before taking the drug.
(1) Persons receiving treatment from a doctor or dentist.
(2) Pregnant women or people who appear to be pregnant.
(3) People who are breastfeeding.
(4) Elderly people.
(5) People who have had allergic symptoms due to medicines, etc.
(6) People who have the following symptoms.
High fever, difficulty urinating
(7) Persons who have received the following diagnosis.
Heart disease, liver disease, high blood pressure, kidney disease, gastric/duodenal ulcer, glaucoma, diabetes, thyroid dysfunction, people with blood clots (cerebral thrombosis, myocardial infarction, thrombophlebitis), people who are at risk of thrombosis.
2. If the following symptoms appear after taking this medicine, it may be a side effect, so stop taking it immediately and consult your doctor, pharmacist, or registered salesperson with this document in hand.
Related parts: Symptoms
Skin: Rash/redness, itching
Gastrointestinal: Nausea/vomiting, loss of appetite, heartburn
Neuropsychiatric system: dizziness
Respiratory: shortness of breath, difficulty breathing
Urinary system: difficulty urinating
Other: Excessive hypothermia
In rare cases, the following serious symptoms may occur. In that case, seek medical treatment immediately.
Symptom name: Symptom
Shock (anaphylaxis): Immediately after taking the drug, symptoms such as itchy skin, hives, hoarseness, sneezing, itchy throat, difficulty breathing, palpitations, and clouded consciousness may occur.
Oculomucocutaneous syndrome (Stevens-Johnson syndrome), toxic epidermal necrolysis, acute generalized exanthematous pustulosis: High fever, bloodshot eyes, eye discharge, sore lips, sore throat, extensive skin rash/redness, small bumps (pustules) appearing on reddened skin, general malaise, loss of appetite, etc. that persist or worsen rapidly.
Liver dysfunction: Fever, itching, rash, jaundice (yellowing of the skin and whites of the eyes), brown urine, general malaise, loss of appetite, etc. may appear.
Kidney disorder: Fever, rash, decreased urine output, general swelling, general malaise, joint pain (pain in the joints), diarrhea, etc. may appear.
Interstitial pneumonia: When you climb stairs or push yourself too hard, you may experience shortness of breath, difficulty breathing, dry cough, fever, etc., which may appear suddenly or persist.
Asthma: wheezing, whistling, and difficulty breathing when breathing.
Aplastic anemia: Bruises, nosebleeds, bleeding gums, fever, pale skin and mucous membranes, fatigue, palpitations, shortness of breath, feeling sick and dizzy, blood in the urine, etc. may appear.
Agranulocytosis: Sudden onset of high fever, chills, sore throat, etc.
3. The following symptoms may appear after taking this medicine. If these symptoms persist or worsen, stop taking this medicine and consult your doctor, pharmacist, or registered salesperson with this document.
Diarrhea, dry mouth, drowsiness
4. If your symptoms do not improve after taking the drug 5 to 6 times, stop taking it and consult your doctor, pharmacist, or registered salesperson with this document.
Precautions for storage and handling
(1) Store tightly closed in a cool, dry place away from direct sunlight.
(2) Store out of reach of children.
(3) Do not transfer to another container (this may cause misuse or change in quality).
(4) Throw away the stuffing inside the bottle after opening the lid. (If you put the stuffing back into the bottle, it will contain moisture and the quality will change. The stuffing is to prevent the tablet from being damaged during transportation.)
(5) Tightly close the lid of the bottle each time you take the medicine (it absorbs moisture and changes its quality).
(6) Do not take products that have passed their expiration date.
(7) Write the date on which the bottle was opened in the “Date opened” field on the box and bottle.
(8) Once opened, in order to maintain quality, take the medicine as soon as possible, preferably within 6 months from the date of opening.
Contact information
Alinamin Pharmaceutical Co., Ltd. “Customer Consultation Office”
23rd floor, Steel Building, 1-8-2 Marunouchi, Chiyoda-ku, Tokyo
0120-567-087
Reception hours
9:00 – 17:00 (excluding Saturdays, Sundays and holidays)
Product size
Height 82mm x Width 46mm x Depth 46mm
| Dimensions | 4.6 × 4.6 × 8.2 cm |
|---|---|
| Medicine Category |
2 |
| Brand |
Benza Ace |
| Manufacturer |
Alinamin Pharmaceutical |
Before using this medicine, be sure to tell your doctor and pharmacist
・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
・If you are a patient with aspirin-induced asthma, or have a history of aspirin-induced asthma.
・If you have a history of hypersensitivity to tiaprofenic acid, suprofen, fenofibrate, or products containing oxybenzone or octocrylene (sunscreen, fragrance, etc.).
・If you have a history with photosensitivity reaction.
・If you are a patient with bronchial asthma.
・If you are under the age of 20 (If you have many opportunities to be exposed to sunlight such as outdoor activities, etc.).
・If you are pregnant or breastfeeding.
・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Precautions while taking this medicine
・Shield the application site from sunlight with colored clothes or supporters during application and for the time being after use, since this medicine may cause photosensitivity (a rash developed at the site exposed to sunlight).