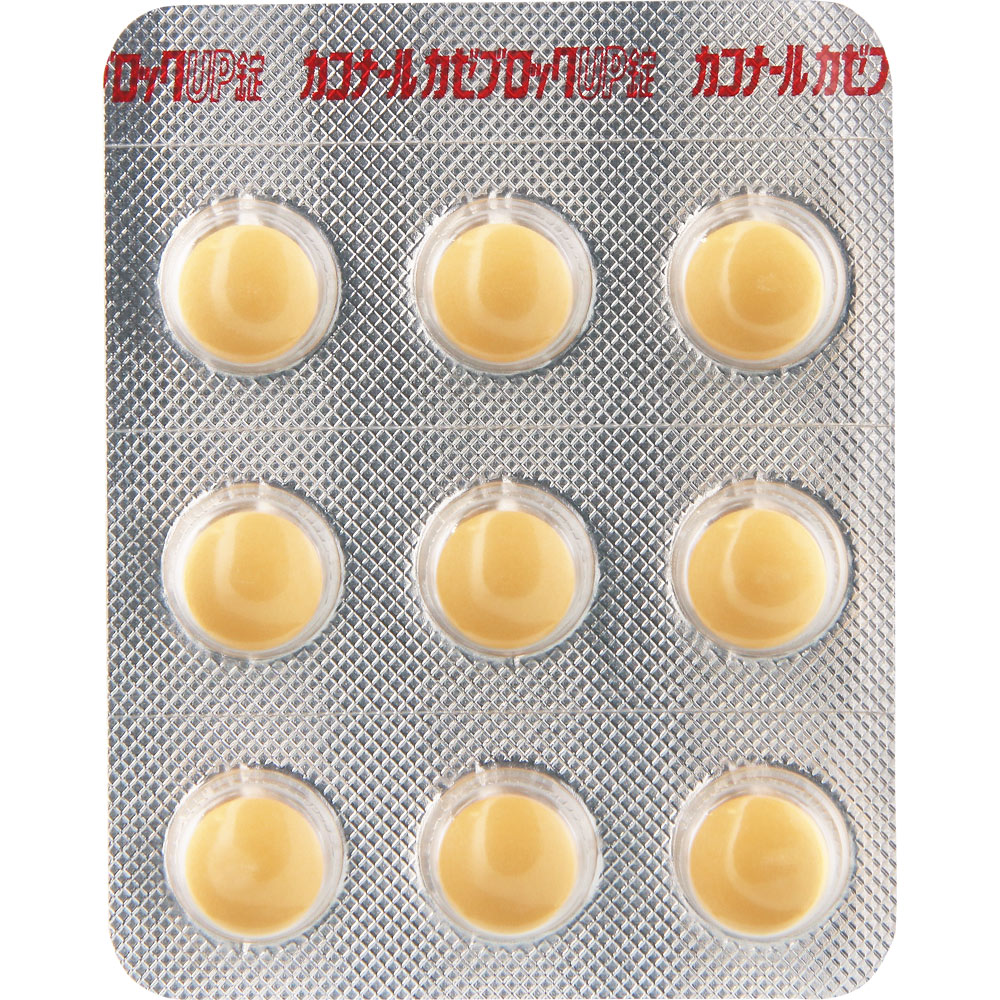
Kaconal Kazeblock Up Tablets 54 Tablets Daiichi Sankyo Healthcare [Designated Class 2 Pharmaceutical
¥2033
This is a cold medicine that has been devised to dissolve ibuprofen quickly, which is effective in reducing fever and analgesia and suppressing inflammation of the airway (throat) mucous membranes (Note).
In addition, the six types of ingredients work in a well-balanced manner and are highly effective in alleviating symptoms such as sore throat, fever, cough, and runny nose caused by colds.
It is a film-coated tablet that is small and easy to swallow.
(Note) Manufacturing process patent No. 3290970 Solid preparation containing poorly soluble NSAIDs (ibuprofen)
Efficacy/effect
[Efficacy/effect]
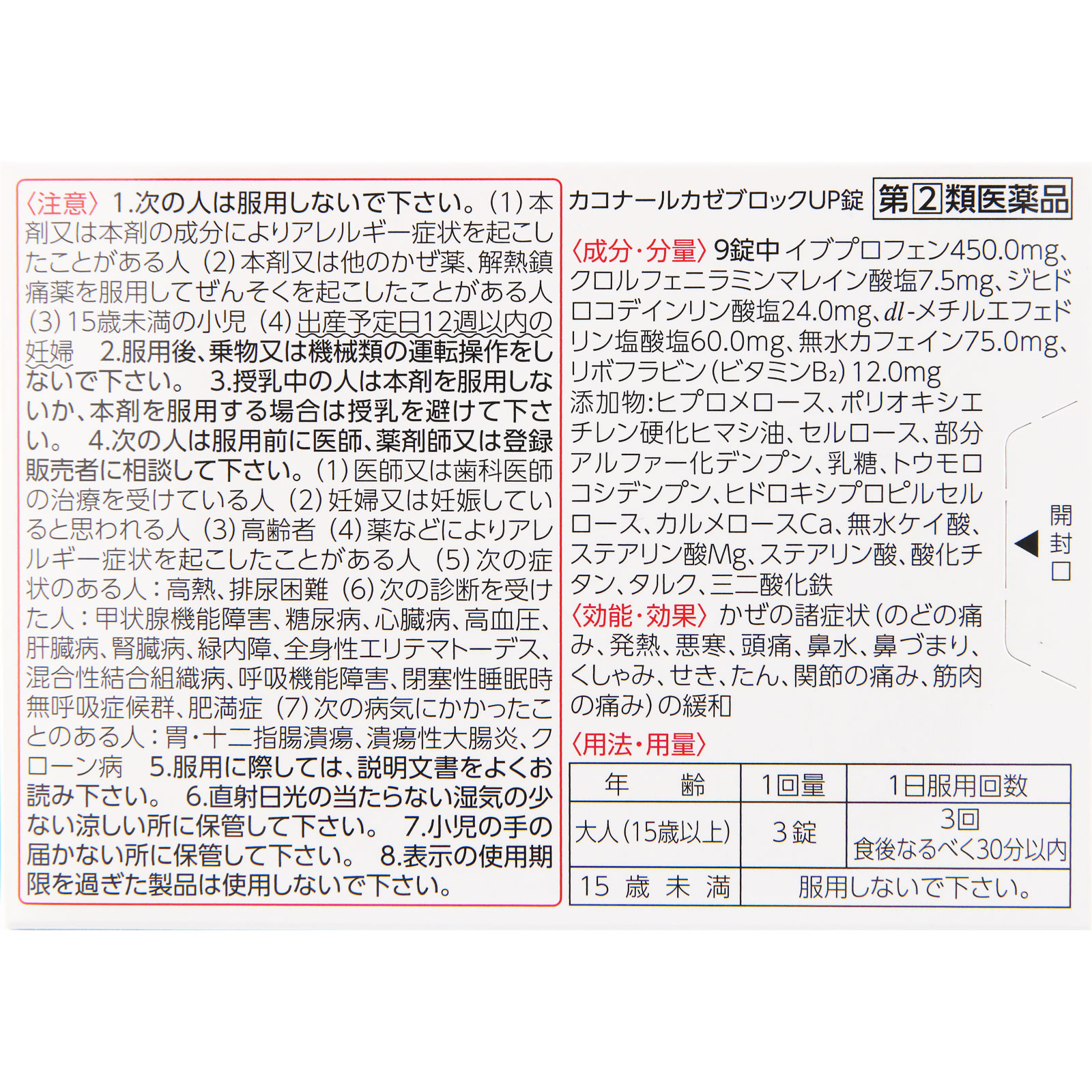
Relief of cold symptoms (sore throat, fever, chills, headache, runny nose, stuffy nose, sneezing, cough, phlegm, joint pain, muscle pain)
Ingredients/Amount
[Ingredients]
9 tablets in total
Ibuprofen: 450mg
(Relieves fever and pain.)
Chlorpheniramine maleate: 7.5mg
(It relieves allergy symptoms such as runny nose and stuffy nose.)
Dihydrocodeine phosphate: 24mg
(Relieves cough.)
dl-methylephedrine hydrochloride: 60mg
(Relieves cough and phlegm.)
Anhydrous caffeine: 75mg
(It relieves headaches.)
Riboflavin (vitamin B2): 12mg
(Replenishes vitamins that tend to be depleted.)
Additives: Hypromellose, polyoxyethylene hydrogenated castor oil, cellulose, partially pregelatinized starch, lactose, corn starch, hydroxypropyl cellulose, carmellose Ca, silicic anhydride, Mg stearate, stearic acid, titanium oxide, talc, iron sesquioxide
(Note regarding ingredients and amounts)
The riboflavin (vitamin B2) contained in this drug may turn your urine yellow.
Usage and dosage
[Dosage/Dosage]
Please take the following amount with water or hot water.
(Age: Dose: Number of doses per day)
Adults (15 years and older): 3 tablets: 3 times (preferably within 30 minutes after meals)
Under 15 years of age: Do not take.
(1) Please strictly follow the directions and dosage.
(2) How to take out the tablet
Press the convex part of the PTP sheet containing the tablet firmly with your fingertips to break the aluminum foil on the back, take it out, and take it.
(If you accidentally swallow it whole, it may pierce the esophageal mucosa or cause an unexpected accident.)
Dosage form/shape
Film coated tablet
Usage precautions
[Precautions for use]
(If you do not follow these guidelines, your current symptoms may worsen or side effects/accidents may occur more easily.)
1. Please do not take this medicine for the following people:
(1) Persons who have had allergic symptoms due to this drug or its ingredients
(2) People who have had asthma while taking this drug or other cold medicines or antipyretic analgesics.
(3) Children under 15 years old
(4) Pregnant women within 12 weeks of due date
2. Do not use any of the following medicines while taking this drug.
Other cold medicines, antipyretic analgesics, sedatives, antitussive expectorants, oral medicines containing antihistamines, etc. (oral medicines for rhinitis, motion sickness medicine, allergy medicines, etc.)
3. Do not operate a vehicle or operate machinery after taking this medicine. (You may experience drowsiness, etc.)
4. Do not take this drug if you are breastfeeding, or avoid breastfeeding if you are taking this drug.
5. Do not drink alcohol before or after taking this medicine.
Do not take for more than 6.5 days.
Consultations regarding use
[Consultations regarding use]
1. The following people should consult a doctor, pharmacist, or registered salesperson before taking this medicine.
(1) Persons receiving treatment from a doctor or dentist.
(2) Pregnant women or people who appear to be pregnant.
(3) Elderly people
(4) People who have had allergic symptoms due to medicines, etc.
(5) People with the following symptoms: high fever, difficulty urinating
(6) Persons with the following diagnoses: thyroid dysfunction, diabetes, heart disease, hypertension, liver disease, kidney disease, glaucoma, systemic lupus erythematosus, mixed connective tissue disease, respiratory dysfunction, obstructive sleep apnea syndrome, obesity
(7) Persons who have had the following diseases: gastric/duodenal ulcer, ulcerative colitis, Crohn’s disease
2. If the following symptoms appear after taking this medicine, it may be a side effect, so please stop taking it immediately and consult your doctor, pharmacist, or registered salesperson with this document.
(Related area: Symptoms)
Skin: Rash/redness, itching, and bruising.
Gastrointestinal: Nausea/vomiting, loss of appetite, stomach discomfort, stomach pain, stomatitis, heartburn, heaviness in the stomach, gastrointestinal bleeding, abdominal pain, diarrhea, bloody stools
Neuropsychiatric system: dizziness
Cardiovascular: palpitations
Respiratory: shortness of breath
Urinary system: difficulty urinating
Other: Blurred vision, ringing in the ears, swelling, nosebleeds, bleeding gums, difficulty in stopping bleeding, bleeding, back pain, excessive drop in body temperature, feeling tired
In rare cases, the following serious symptoms may occur: In that case, please see a doctor immediately.
(Symptom name: Symptom)
Shock (anaphylaxis): Immediately after taking the drug, symptoms such as itchy skin, hives, hoarseness, sneezing, itchy throat, difficulty breathing, palpitations, and clouded consciousness may occur.
Oculomucocutaneous syndrome (Stevens-Johnson syndrome): High fever, bloodshot eyes, mucus, sore lips, sore throat, and widespread skin rash/redness that persist or worsen rapidly.
Toxic epidermal necrolysis: High fever, bloodshot eyes, mucus, sore lips, sore throat, and widespread rash/redness on the skin persist or worsen rapidly.
Liver dysfunction: Fever, itching, rash, jaundice (yellowing of the skin and whites of the eyes), brown urine, general malaise, loss of appetite, etc. may appear.
Kidney disorder: Fever, rash, decreased urine output, general swelling, general malaise, joint pain (pain in the joints), diarrhea, etc. may appear.
Aseptic meningitis: Severe headache accompanied by stiffness in the neck, fever, nausea, and vomiting appear. (Such symptoms are particularly frequently reported in people receiving treatment for systemic lupus erythematosus or mixed connective tissue disease.)
Interstitial pneumonia: When you climb stairs or push yourself too hard, you may experience shortness of breath, difficulty breathing, dry cough, fever, etc., which may appear suddenly or persist.
Asthma: wheezing, whistling, and difficulty breathing when breathing.
Aplastic anemia: Bruises, nosebleeds, bleeding gums, fever, pale skin and mucous membranes, fatigue, palpitations, shortness of breath, feeling sick and dizzy, blood in the urine, etc. may appear.
Agranulocytosis: Sudden onset of high fever, chills, sore throat, etc.
Respiratory depression: Shortness of breath, difficulty breathing, etc. appear.
3. The following symptoms may appear after taking this medicine. If these symptoms persist or worsen, please stop taking this medicine and consult your doctor, pharmacist, or registered salesperson with this document.
Constipation, dry mouth, drowsiness
4. If your symptoms do not improve after taking this medicine 5 to 6 times, please stop taking it and consult your doctor, pharmacist, or registered salesperson with this document. (Especially when the fever lasts for more than 3 days or occurs repeatedly)
Precautions for storage and handling
(1) Store in a cool, dry place away from direct sunlight.
(2) Please keep out of reach of children.
(3) Please do not replace it with another container. (This may cause misuse or change in quality)
(4) Do not use products whose expiration date has passed.
Contact information
Daiichi Sankyo Healthcare Co., Ltd. Customer Service Office
3-14-10 Nihonbashi, Chuo-ku, Tokyo 103-8234
0120-337-336
Product size
Height 70mm x Width 100mm x Depth 27mm
| Dimensions | 2.7 × 10 × 7 cm |
|---|---|
| Medicine Category |
2 |
| Brand |
Kakonal |
| Manufacturer |
Daiichi Sankyo Healthcare |
Before using this medicine, be sure to tell your doctor and pharmacist
・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
・If you are a patient with aspirin-induced asthma, or have a history of aspirin-induced asthma.
・If you have a history of hypersensitivity to tiaprofenic acid, suprofen, fenofibrate, or products containing oxybenzone or octocrylene (sunscreen, fragrance, etc.).
・If you have a history with photosensitivity reaction.
・If you are a patient with bronchial asthma.
・If you are under the age of 20 (If you have many opportunities to be exposed to sunlight such as outdoor activities, etc.).
・If you are pregnant or breastfeeding.
・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Precautions while taking this medicine
・Shield the application site from sunlight with colored clothes or supporters during application and for the time being after use, since this medicine may cause photosensitivity (a rash developed at the site exposed to sunlight).