Kewpie Kowa Chondroizer Α 180 Tablets Kowa [Class 2 Drug]
¥7236
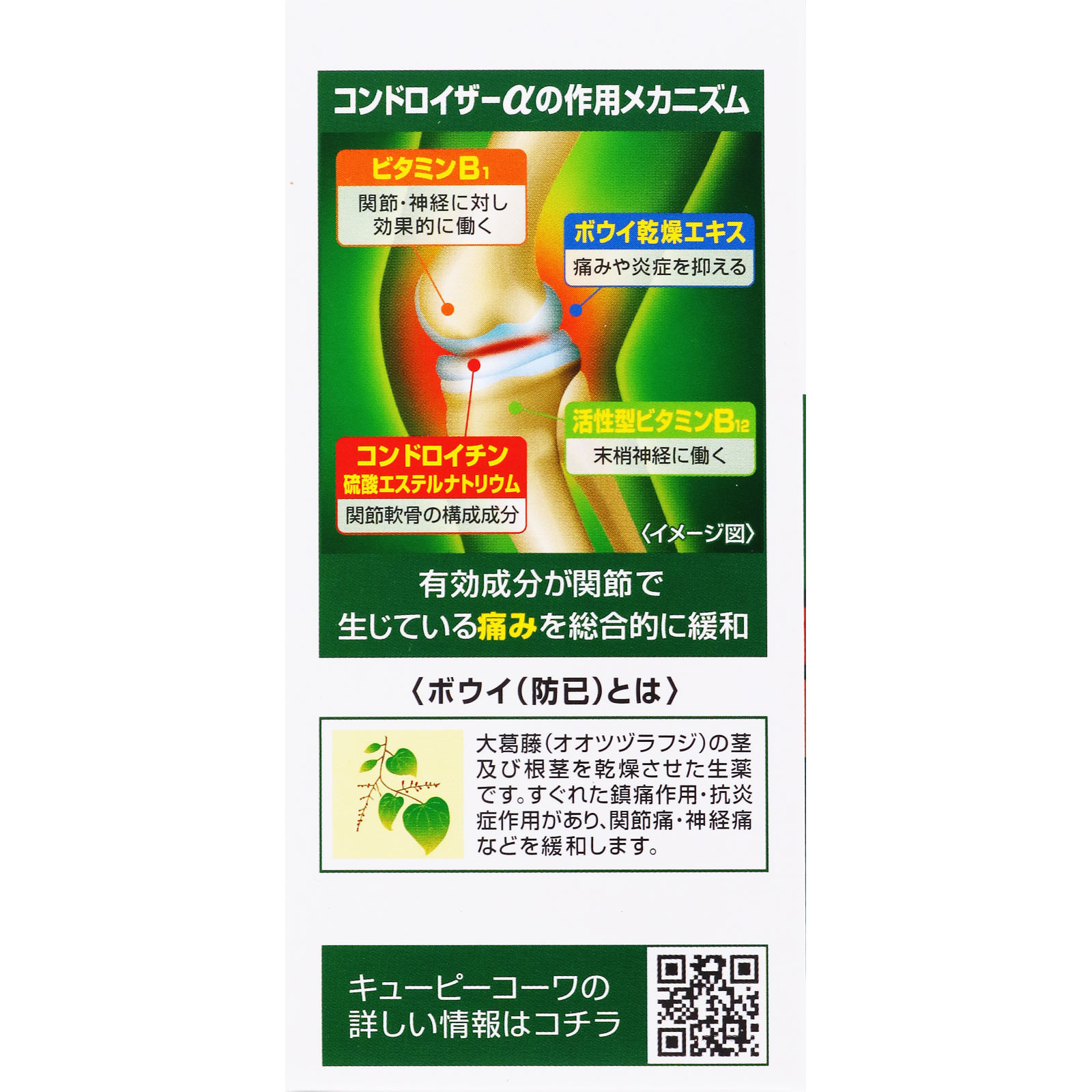
Continuously contains Bowie dry extract, which has anti-inflammatory and analgesic effects.
Added anti-inflammatory gamma oryzanol!
Changed cyanocobalamin (VB12) to active type mecobalamin!
Increase the amount of chondroitin sulfate Na to the standard* maximum amount!
The five active ingredients are
of joints such as knees
A wide range of pain relief!
Efficacy/effect
1. Relief of the following symptoms: joint pain/muscle pain (shoulder/hip/elbow/knee pain, stiff shoulders, frozen shoulders, etc.), neuralgia, numbness in the limbs, constipation, eye strain (chronic eye fatigue and associated blurred vision/pain behind the eyes)
2. Beriberi: However, if symptoms 1 and 2 do not improve after using this product for about a month, consult your doctor or pharmacist.
3. Supplementation of vitamin B1 in the following cases: when physically fatigued, during pregnancy/lactation, when physical strength decreases during or after illness
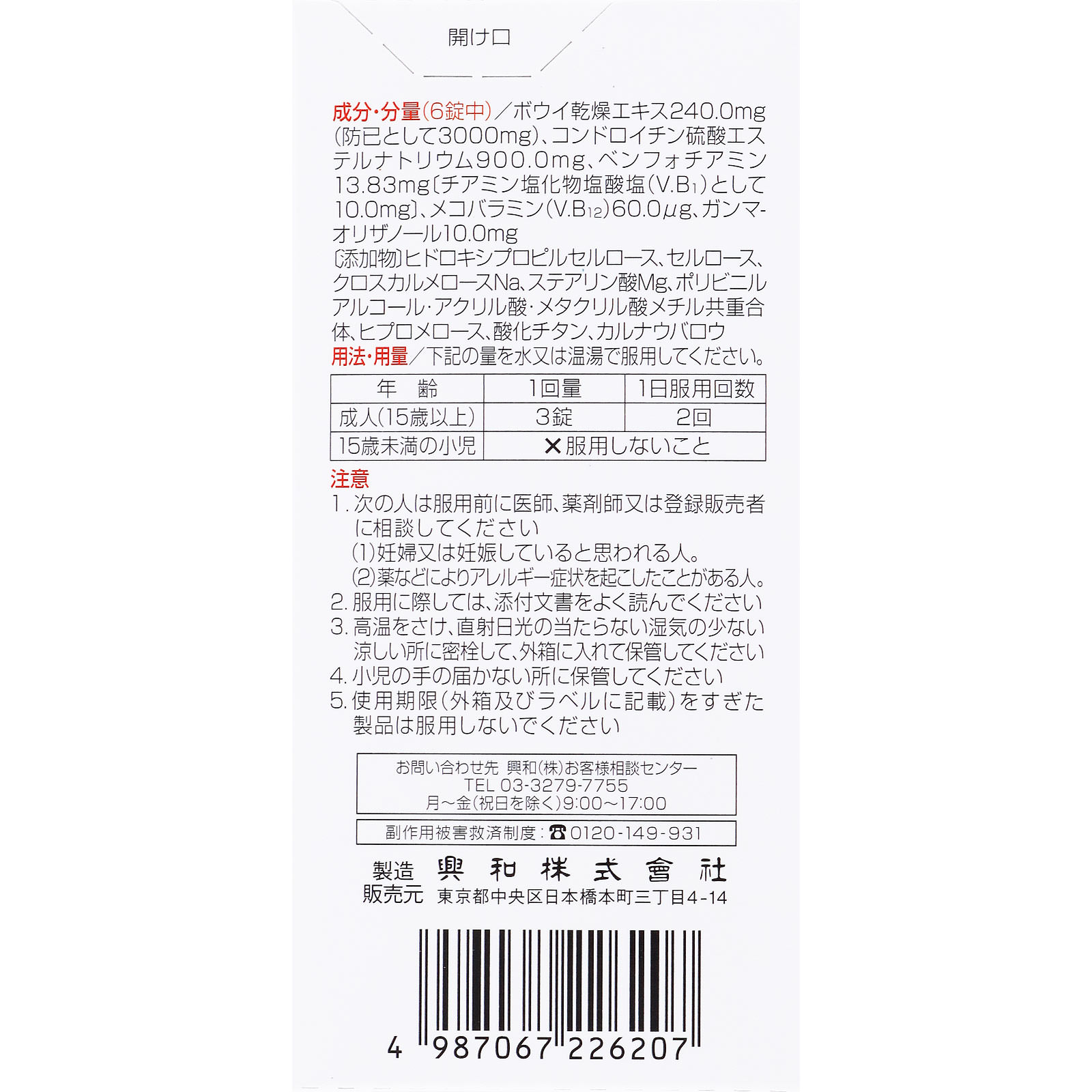
Ingredients/Amount
(Daily dose/6 tablets)
Bowie dried extract
[As a barrier]
240.0mg
(3000mg)
Chondroitin sulfate sodium
900.0mg
benfotiamine
[As thiamine chloride hydrochloride (V.B1)]
13.83mg
(10.0mg)
Mecobalamin (V.B12)
60.0μg
Gamma-oryzanol
10.0mg
[Additives] Hydroxypropyl cellulose, cellulose, croscarmellose Na, Mg stearate, polyvinyl alcohol/acrylic acid/methyl methacrylate copolymer, hypromellose, titanium oxide, carnauba wax
Usage and dosage
Please take the following amount with water or warm water.
●Can be taken at any time, before or after meals.
Adults (15 years and older): 3 tablets per dose, 2 times per day
Children under 15 years of age: Do not take
[Precautions regarding usage and dosage]
Please strictly follow the directions and dosage.
Usage precautions
-
Consultations regarding use
1. The following people should consult a doctor, pharmacist, or registered salesperson before taking the drug.
(1) Pregnant women or people who appear to be pregnant.
(2) People who have had allergic symptoms due to drugs, etc.
2. If the following symptoms appear after taking this medicine, it may be a side effect, so please stop taking it immediately and consult your doctor, pharmacist, or registered salesperson with this package insert.
Related parts/symptoms
Skin/rash/redness, itching
Digestive system/nausea/vomiting, loss of appetite
3. The following symptoms may appear after taking this medicine. If these symptoms persist or worsen, please stop taking this medicine and consult your doctor, pharmacist, or registered salesperson with this package insert.
loose stools, diarrhea
4. If your symptoms do not improve after taking this medicine for about a month, please stop taking it and consult your doctor, pharmacist, or registered salesperson with this package insert.
Precautions for storage and handling
1. Avoid high temperatures and store in a cool, dry place out of direct sunlight, tightly closed and in the outer box. (Light may affect quality.)
2. Please keep out of reach of children.
3. Do not transfer to another container. (It may cause misuse or change in quality.)
4. If water gets on the tablet, the contents may change, so do not drop water droplets on it or touch it with wet hands. If you accidentally get the tablet wet, discard the wet tablet.
5. The packing material (vinyl) inside the container is placed to prevent the tablet from being damaged during transportation, so be sure to throw it away after opening the cap.
6. If the cap of the container is not tightly tightened, moisture may affect the quality of the medicine, so please tighten the cap thoroughly each time you take the medicine.
7. Please write the date you opened the cap in the “Date opened” field on the outer box and label.
8. Do not take the product after the expiration date (written on the outer box and label). Also, once you open the cap, please use it within 6 months from the date of opening to ensure quality.
Contact information
Kowa Co., Ltd. Pharmaceutical Division Customer Consultation Center
103-8433 3-4-14 Nihonbashi Honmachi, Chuo-ku, Tokyo
03-3279-7755
Product size
Height 110mm x Width 52mm x Depth 52mm
| Dimensions | 5.2 × 5.2 × 11 cm |
|---|---|
| Medicine Category |
2 |
| Brand |
Kewpie Kowa |
| Manufacturer |
Kowa |
Before using this medicine, be sure to tell your doctor and pharmacist
・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
・If you are a patient with aspirin-induced asthma, or have a history of aspirin-induced asthma.
・If you have a history of hypersensitivity to tiaprofenic acid, suprofen, fenofibrate, or products containing oxybenzone or octocrylene (sunscreen, fragrance, etc.).
・If you have a history with photosensitivity reaction.
・If you are a patient with bronchial asthma.
・If you are under the age of 20 (If you have many opportunities to be exposed to sunlight such as outdoor activities, etc.).
・If you are pregnant or breastfeeding.
・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Precautions while taking this medicine
・Shield the application site from sunlight with colored clothes or supporters during application and for the time being after use, since this medicine may cause photosensitivity (a rash developed at the site exposed to sunlight).