Matsukiyo Lacour Tape Fb 5.0Ex 10 Pieces [Class 2 Drugs]
¥966
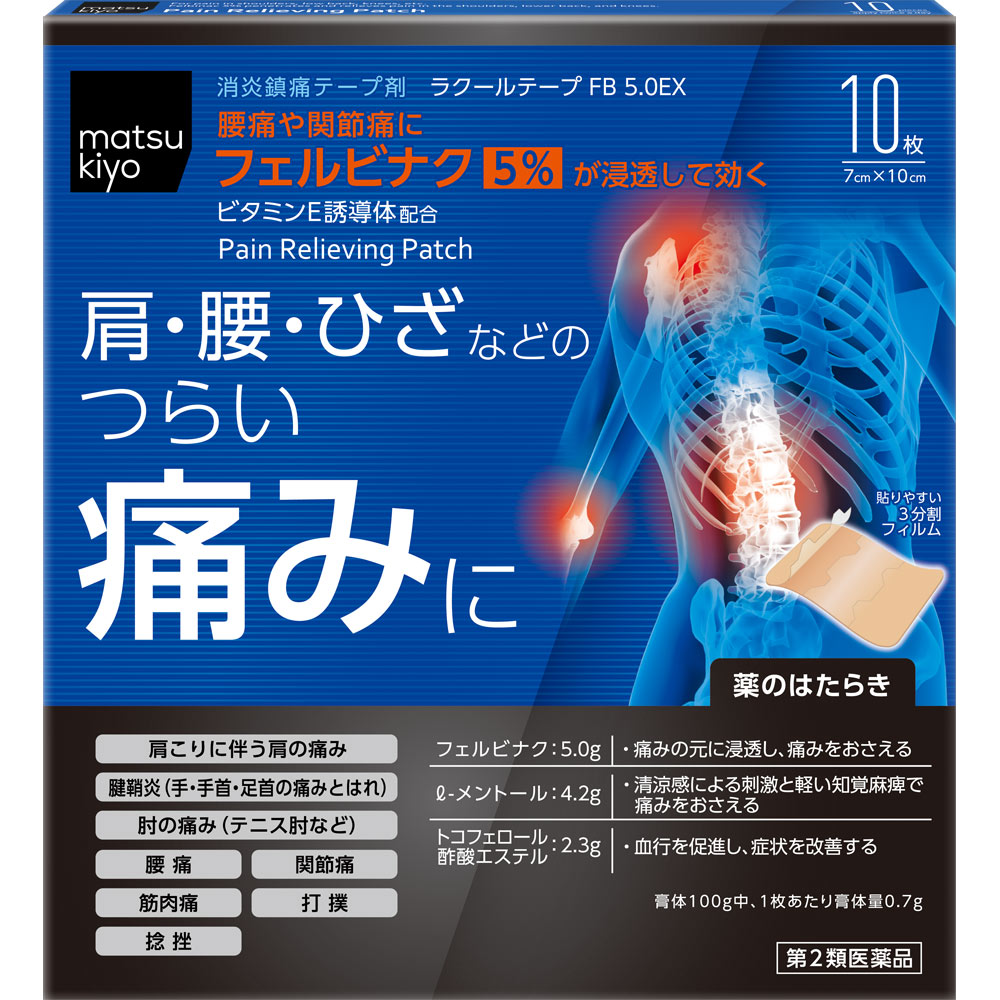
1. Felbinac acts directly on pain in the shoulders and lower back.
2. The refreshing feeling has been improved by increasing the amount of l-menthol.
3. Newly formulated tocopherol acetate promotes blood circulation.
Efficacy/effect
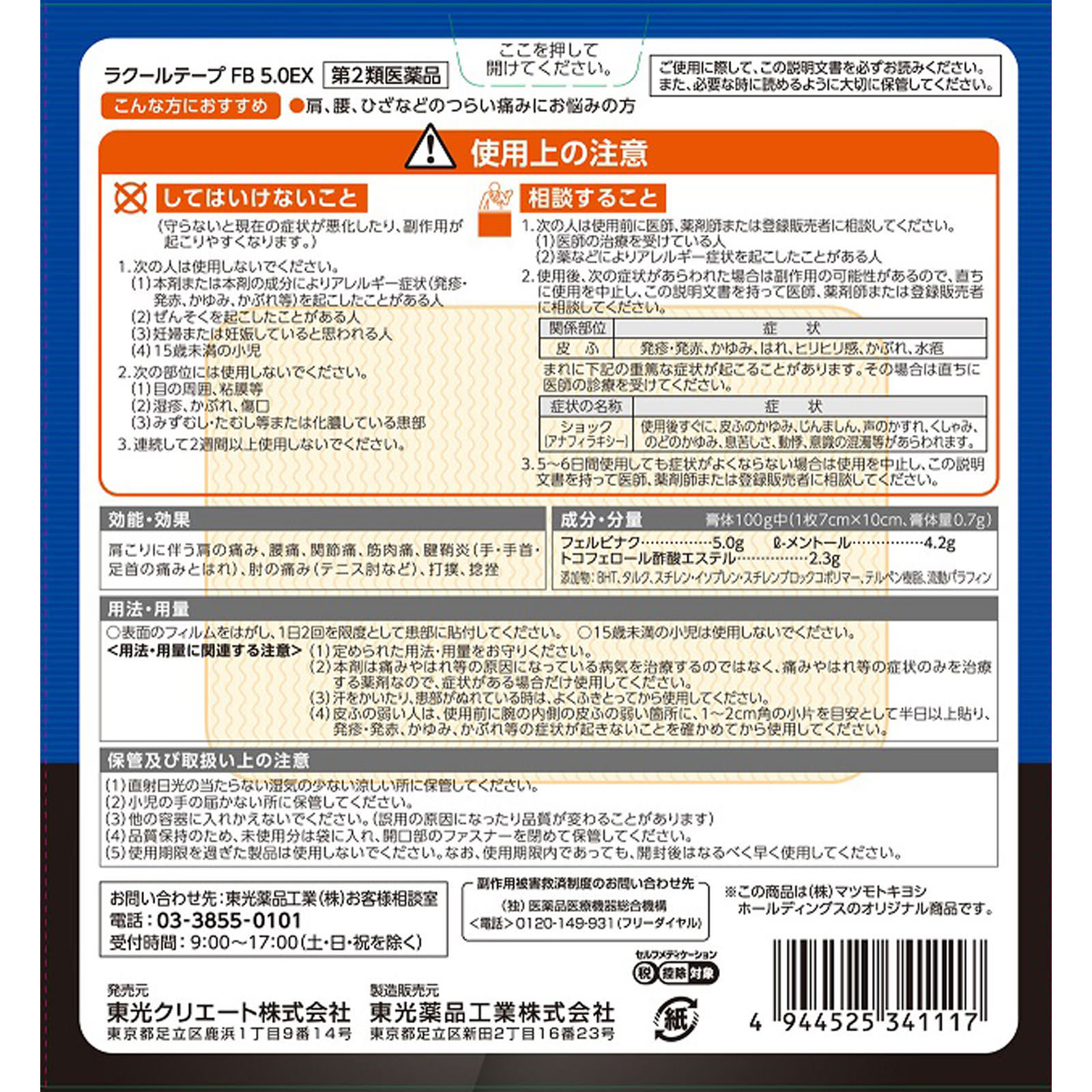
Shoulder pain associated with stiff shoulders, lower back pain, joint pain, muscle pain, tendonitis (pain and swelling in the hands, wrists, and ankles), elbow pain (tennis elbow, etc.), bruises, sprains
Ingredients/Amount
In 100g of plaster: 5.0g of felbinac, 4.2g of l-menthol, 2.3g of tocopherol acetate
Usage and dosage
Peel off the film on the surface and apply it to the affected area at most twice a day.
Dosage form/shape
Tape agent
Usage precautions
1. Do not use the following persons
(1) Persons who have experienced allergic symptoms (rash/redness, itching, rash, etc.) due to this drug or its ingredients
(2) People who have had asthma
(3) Pregnant women or those who appear to be pregnant.
(4) Children under 15 years old
2. Do not use on the following areas
(1) Around the eyes, mucous membranes, etc.
(2) Eczema, rash, wounds
(3) Affected areas with athlete’s foot, ringworm, etc. or suppuration.
3. Do not use it continuously for more than 2 weeks.
Consultations regarding use
1. The following people should consult a doctor, pharmacist, or registered salesperson before use.
(1) Persons receiving treatment from a doctor
(2) People who have had allergic symptoms due to drugs, etc.
2. If the following symptoms appear after use, it may be a side effect, so stop using it immediately and consult your doctor, pharmacist, or registered seller with this instruction sheet. Related areas, skin, symptoms, rash/redness, itching, swelling, stinging, rash, blisters In rare cases, the following serious symptoms may occur. In that case, please see a doctor immediately. Symptom name, anaphylaxis, symptoms, Itchy skin, hives, hoarseness of voice, sneezing, itchy throat, difficulty breathing, palpitations, clouded consciousness, etc. may appear immediately after use.
3. If your symptoms do not improve after using this product for 5 to 6 days, please stop using it and consult your doctor, pharmacist, or registered salesperson with this instruction sheet.
Precautions for storage and handling
(1) Store in a cool, dry place away from direct sunlight.
(2) Please keep out of reach of children.
(3) Do not transfer to another container. (This may cause misuse or change in quality.)
(4) To maintain quality, store unused portions in a bag with the opening closed.
(5) Do not use products that have passed their expiration date. Please use the product as soon as possible after opening, even if it is within the expiration date.
Contact information
Toko Pharmaceutical Co., Ltd.
1-9-14 Shikahama, Adachi-ku, Tokyo
03-3855-0101
Product size
Height 160mm x Width 148mm x Depth 20mm
| Dimensions | 2 × 14.8 × 16 cm |
|---|---|
| Medicine Category |
2 |
| Brand |
Matsukiyo |
Before using this medicine, be sure to tell your doctor and pharmacist
・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
・If you are a patient with aspirin-induced asthma, or have a history of aspirin-induced asthma.
・If you have a history of hypersensitivity to tiaprofenic acid, suprofen, fenofibrate, or products containing oxybenzone or octocrylene (sunscreen, fragrance, etc.).
・If you have a history with photosensitivity reaction.
・If you are a patient with bronchial asthma.
・If you are under the age of 20 (If you have many opportunities to be exposed to sunlight such as outdoor activities, etc.).
・If you are pregnant or breastfeeding.
・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Precautions while taking this medicine
・Shield the application site from sunlight with colored clothes or supporters during application and for the time being after use, since this medicine may cause photosensitivity (a rash developed at the site exposed to sunlight).